-
Nath: high demand for syringes
Injection protocols
Along with this, there is a mechanism to observe injection protocols and safe disposal of waste items such as used syringes and needles. Likewise, all reports of adverse reactions or other medical problems associated with the vaccination would be recorded and integrated with the CO-WIN software. Until date, the software has created records of 7.5 million beneficiaries registered with the authorities.
An important consideration at the planning level is the maintenance of the vaccine stocks at low temperatures of 2-8 degrees Celsius with the help of the cold chain mechanism. This is done through a series of refrigeration and low temperature containers, many of which can travel through long distances. Since there is a chance that vaccine stocks from India may be exported to other countries as well, this system becomes even more crucial. In case the cold chain happens to break even for short periods, the effectiveness of the vaccine can no longer be guaranteed.
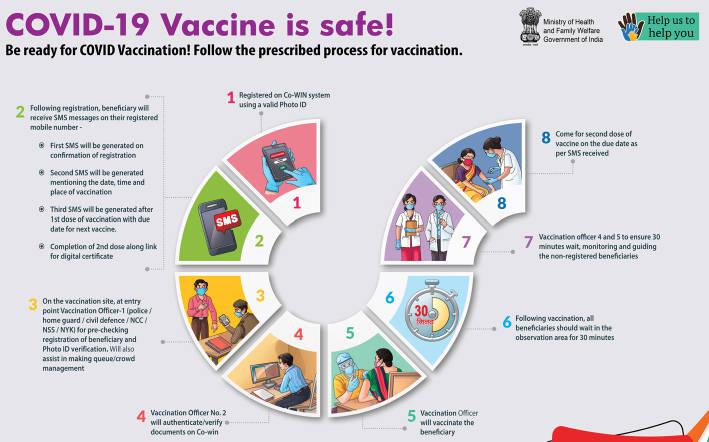
To ensure that everything happens according to plan, the union government along with the various states has staged a series of rehearsals during the first week of January 2021. In the first such exercise, held on 29 December, four states were covered: Assam, Andhra Pradesh, Punjab and Gujarat. The actual locations were: Krishna district (Andhra Pradesh), Rajkot and Gandhinagar (Gujarat), Ludhiana and Shaheed Bhagat Singh Nagar (Nawanshahr, Punjab) and Sonitpur and Nalbari districts (Assam).
As part of the exercise, the district administration of each location formed special teams that used dummy beneficiary data, vaccination site creation, allocated of vaccine stocks and practiced communication of all relevant details to the vaccinators. The details were recorded n the CO-WIN software, which also incorporated suggestions from the field workers.
In the second round, conducted on 8 January, the exercise was expanded to cover all the states and Union Territories (except Uttar Pradesh and Haryana, which were covered on different days). Alongside, the necessary training was imparted to 17 lakh vaccinators and 300,000 team members. In this round, each district was asked to identify three types of session sites, including a public health facility such as a District Hospital or Medical College, a private health facility and some rural or urban outreach sites.
The dry run was also intended to familiarise the state, district, block and hospital level officers on all aspects of the Covid-19 roll-out. This activity was supposed to help administrators in strengthening the linkages between planning, implementation and reporting mechanisms, identification of any residual challenges.
Role of the private sector
While all these steps are largely focussed on the public sector in health, private healthcare organisations like FICCI (Federation of Indian Chambers of Commerce and Industry) are also gearing up to play their part. In partnership with Ernst & Young, a major consulting firm, the FICCI Health Services committee has put together a detailed report offering to develop public-private partnerships for tackling Covid-19. “We hope the government has taken note of intent and commitment of the private sector players for accelerating the process of targeted vaccination across the country,” says Alok Roy, chair, FICCI Health Committee.
-

Ella: long and illustrious record
Among the prominent players to participate in the 8 January dry run was the Apollo Hospital in Nungabakkam, Chennai. “It’s a prestigious opportunity to be a part of the entire process which would put us in forefront to vaccinate the frontline workers who are serving our countrymen during the Covid-19 pandemic,” said Preetha Reddy, executive vice-chairperson, Apollo group of Hospitals.
Even as the vaccine stocks are being created in different manufacturing facilities of Serum Institute and other companies in millions of doses every month, other companies including Hindustan Syringes and Medical Devices (HMD) are producing syringes and needles with which to administer the injections.
“The estimated demand in India would be about 900 million pieces of different kinds of syringes for just one shot of the vaccine, considering that 60-70 per cent of the country gets vaccinated,” says Rajiv Nath, managing director, HMD. “The number would amplify to 1.8 billion, if the vaccine India chooses needs two shots”.
The syringes that HMD produces are of the auto-disabled variety, which ensures that each syringe-needle set can be used only once. This helps to maintain hygiene and avoid infection at the injection site, which was a common complication in the bygone era of glass syringes. HMD is one of just four companies recognised for supplying auto-disabled syringes to UNICEF and other international agencies.
Since the Covid-19 pandemic began and prospects of a vaccine becoming available drew close, HMD has supplied 140 million AD syringes through the UNICEF, for global vaccination campaigns. Interestingly, HMD’s auto-disabled syringes have been pre-qualified by the World Health Organisation (WHO). They are also expected to be used for administering the Pfizer vaccine, which requires a 0.3 ml syringe, as opposed to the standard 0.5 ml one.
This vaccination drive will also demonstrate the capacity of the entire country, with its diversity of language, food habits, clothing styles and other aspects to pull together and work as one, whenever we are faced with the threat of a national calamity.
-
Firestorm of criticism
At 11 am on 3 January, the first Sunday of the New Year, the Drug Controller General of India (DCGI), V.G. Somani made a short media statement streamed live on YouTube. Two vaccines against Covid-19, namely Covaxin developed by Bharat Biotech and Covishield, which is to be manufactured and marketed in India by Serum Institute of India (SII) had been granted Emergency Use Authorization (EAU). This means they could be marketed and administered to patients who need it on an urgency basis and cannot wait for the full clinical trials to be completed.
Bharat Biotech “has generated safety and immunogenicity data in various animal species such as mice, rats, rabbits, Syrian hamster, and also conducted challenge studies on non-human primates (Rhesus macaques) and hamsters. All these data has been shared by the firm with CDSCO. Phase-I and Phase-II clinical trials were conducted in approximately 800 subjects and the results have demonstrated that the vaccine is safe and provides a robust immune response,” says a DCGI news release issued the same day. The release also mentions that Bharat Biotech had initiated Phase-III clinical trial (which is the final testing phase before marketing of any medicinal or vaccine product) in about 25,800 human volunteers. The results of this phase are obviously awaited. Similar information was given about the SII vaccine as well.
It is worth noting that the Covaxin is a research product of the National Institute of Virology (NIV), Pune which functions under the umbrella of the Indian Council of Medical Research (ICMR) while Covishield was developed by Oxford University to be marketed internationally by AstraZeneca (Cover Feature, Business India, 27 July-9 August 2020). SII has obtained the requisite licences to make and market Covishield in India and other emerging economies.
But within hours of the DCGI announcement, a barrage criticism of the DCGI decision as well as Bharat Biotech broke out in the media, primarily seizing upon the fact that DCGI had allowed the Covaxin to be administered to people before the trials had been completed. Baseless allegations of undue haste in bringing the product to the market, and ‘government pressure’ on the DCGI to approve it were seen and heard in all kinds of media platforms. The community of scientific researchers was also divided right down the middle.
Eminent scientist Gagandeep Kang claimed that she had not seen any published data on Covaxin and hence its efficacy could not be vouched for. On the other hand, T. Jacob John, former ICMR chief of virology, told some reporters that efficacy was concerned with how long the vaccine’s immunity would last, while there was little doubt that Covaxin did generate some immunity against the Corona Virus soon after it was injected.
A few important points have emerged from this controversy. First, Emergency Authorisation also labeled as Restricted Emergency Use (REU) does not mean the product can be sold freely to each and every one. It is a procedure introduced by the US drug regulators to enable patients at risk of death or serious illness if they are not given the product in question. Hence many developed countries have granted EAU for one or other Covid-19 vaccine. India is not alone.
Second, any company that seeks permission for marketing any product is bound to share the clinical trial data with the regulators of each country. This Bharat Biotech has obviously. “To publish the results or not, even in the academic and scientific press is a prerogative of the company,” says Ganesh Divekar, vice-president, clinical operations & biometrics, Siro Clinpharm, one of the largest clinical trial organisations in India.
Third, Bharat Biotech is hardly a novice in the field of vaccines and biotech products. “Our company has a long and illustrious record of manufacturing vaccines against many diseases and conducts clinical trials all over the world,” pointed out the company’s founder Krishna Ella, in an hour-long media statement on 5 January.
So, why is there a furore against Covaxin and mere whispers about SII? One possibility is that these products are likely to be cheaper than the MNC offerings. Another is that Covaxin is viewed as an offspring of the Modi government and, therefore, targeted by those opposed to it. But these could be mere speculation, completely unsupported by hard facts.





































